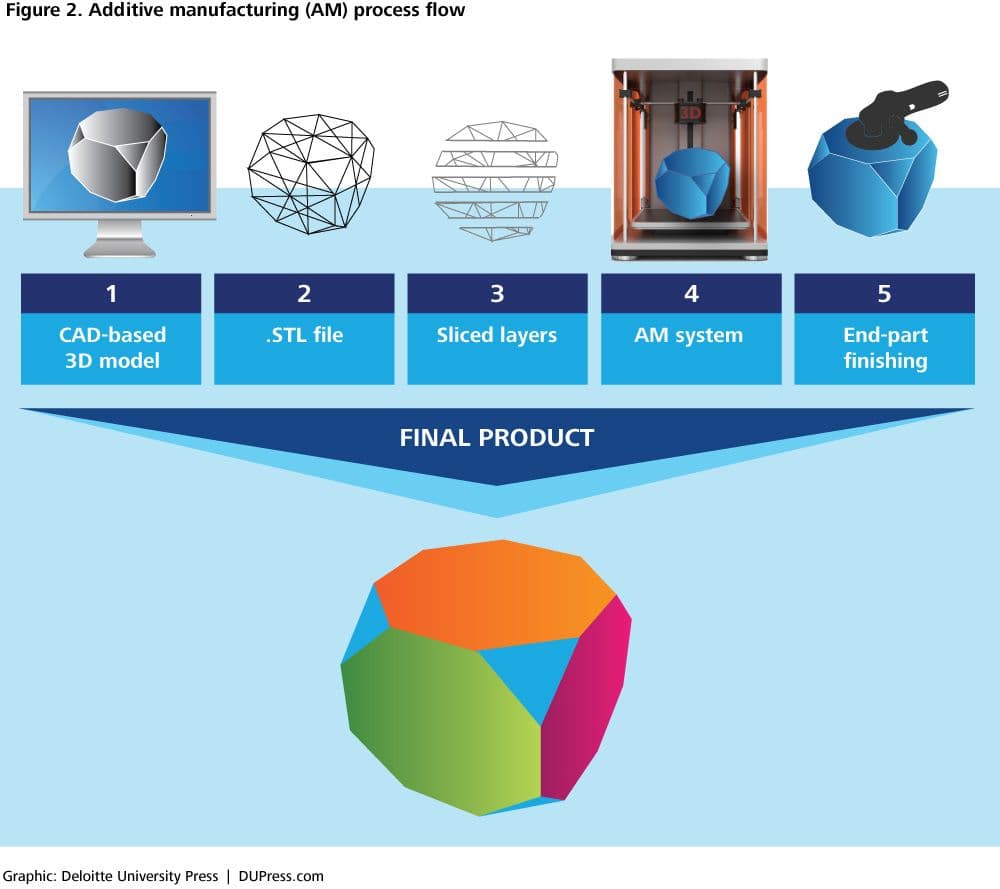
Nivalon unveils world's first patient-specific, metal-free, motion-preserving spinal implant
Nivalon produced a patient-specific, metal-free spinal implant using AI-driven design and ceramic 3D printing, a potential step toward MRI-friendly, custom load-bearing implants.

Nivalon Medical Technologies says it has produced what the company calls the world's first fully patient-specific, motion-preserving spinal implant built entirely without metal. The device pairs zirconia-toughened alumina ceramic endplates with a flexible elastomeric core and is digitally designed from each patient’s CT data using AI-driven tools, a combination aimed at matching individual anatomy and natural biomechanics.
The implant was manufactured using XJet NanoParticle Jetting ceramic 3D printing and, Nivalon stated, represents "a new and distinct microstructural class of biocompatible implant material." The company said that "SEM analysis at UConn confirmed that the printed ZTA ceramic represents a new and distinct microstructural class of biocompatible implant material," and described the ceramic architecture as one that "behaves like bone" while the elastomeric core preserves motion. Nivalon also described the work as "Clinically Validated Through Independent Biomechanical, Mechanical, Biological, and Surgical Testing."
For the additive manufacturing community, the announcement is notable because it showcases a path from lab-scale ceramic prints to load-bearing parts conceived for clinical use. Nivalon framed the milestone as combining patient-specific geometry, custom biomechanics, and load-bearing ceramic parts in a single implant. Industry reporting characterized the effort as marking "a transition from research to clinical manufacturing," while also noting the company is not presenting the device as a finished commercial product.
Nivalon said it holds two issued U.S. patents and has six pending, and that it is preparing for NIH Phase II SBIR funding and an FDA Premarket Approval clinical pathway. The company plans first-in-human procedures in 2026 and explicitly named Todd Hodrinsky, Nivalon co-founder and CEO, among those planned. The work was performed in collaboration with the Youngstown Business Incubator Advanced Manufacturing and Engine Tech programs and used SEM analysis performed at the University of Connecticut.
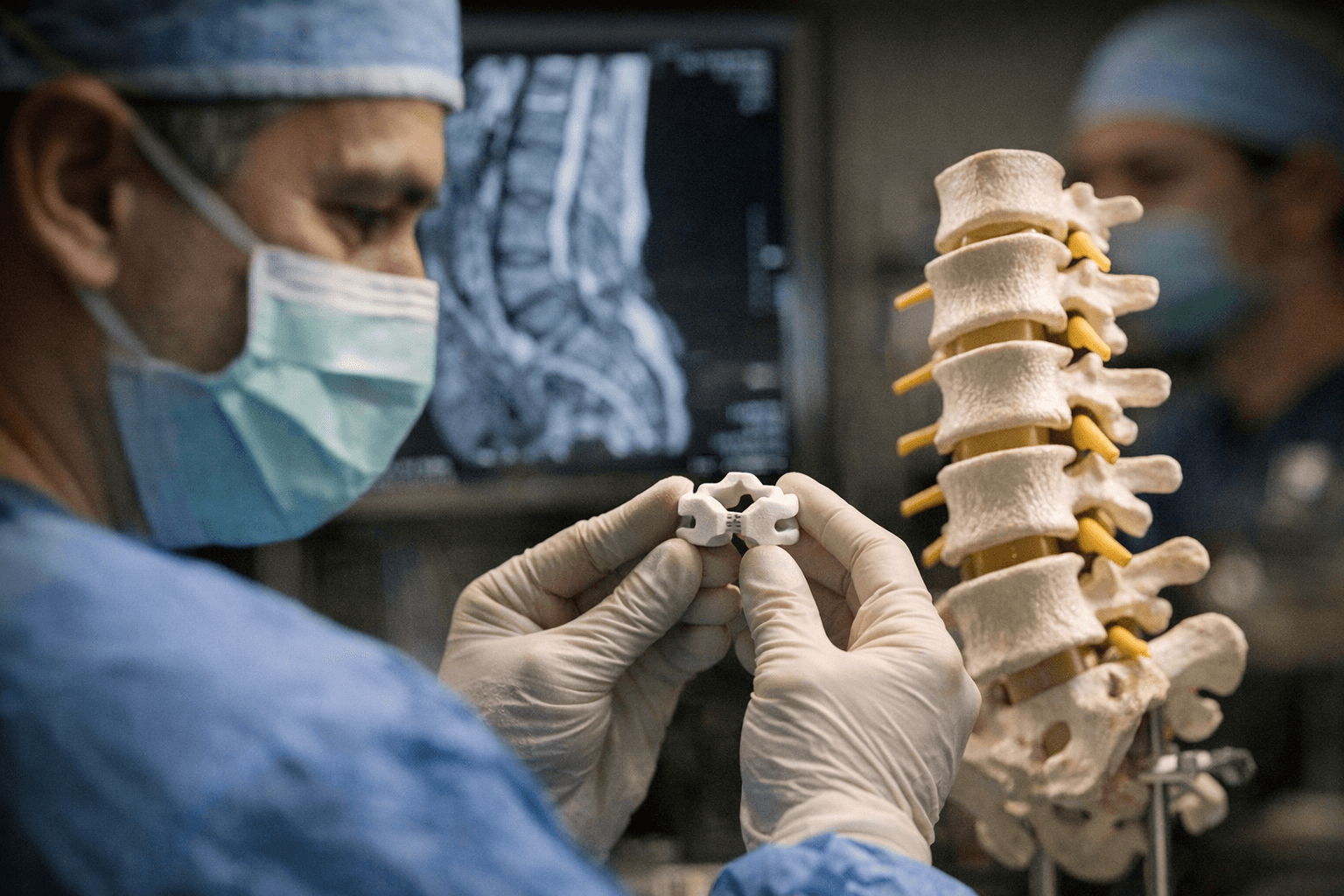
The practical value for makers, service bureaus, and clinical AM shops is clear: ceramic additive technologies like XJet’s NanoParticle Jetting can be pushed toward repeatable, load-bearing medical parts if validation, QA, and regulatory work follow. Potential advantages cited by Nivalon include elimination of metal-related corrosion and ion release, reduced stiffness mismatch, and lower imaging artifacts on MRI and CT.
Significant gaps remain. Full independent test reports, peer-reviewed data, patent documentation, manufacturing throughput and quality metrics, and formal FDA filings have not been released publicly. Nivalon’s production claim establishes feasibility; clinical safety, long-term performance, and regulatory clearance will determine whether this print becomes standard practice. For now, the additive manufacturing community should watch for the promised clinical data, SEM reports, and regulatory milestones that will either validate or temper this ambitious first step.
Know something we missed? Have a correction or additional information?
Submit a Tip